Electron Pair Geometry For Nh3
Geometry in chemistry refers to the shape of molecules in 3-Dimensional space.
Molecular geometry is described every bit the 3D organisation of atoms in a molecule, usually relative to a single central cantlet. Whereas, electron geometry is the 3D system of electron pairs effectually a primal atom, whether bonding or not-bonding.
A lone (not-bonding) pair refers to a pair of valence electrons that are non shared with another atom in a covalent bail, while a bail pair is a pair of electrons nowadays in a bond.
Information technology is well known that the electron pairs, beingness negatively charged, repel each other. This repulsion causes the electron pairs around the central atom to adapt as far apart from each other as possible. This minimizes the repulsion.
Under the influence of a single nucleus, a lone pair offers more repulsion than a bond pair which is influenced by two nuclei. This causes a slight decrease in bond angles (angles between bonds or bail pairs).
If all of the electron groups are bond pairs (no lone pairs), the molecular geometry and electron geometry are the same. An instance is a methyl hydride molecule, CH4 with 4 bond pairs and no solitary pairs, all 4 of carbon's valence electrons are bonded with hydrogen atoms. Its molecular, every bit well equally electronic geometry, is tetrahedral.
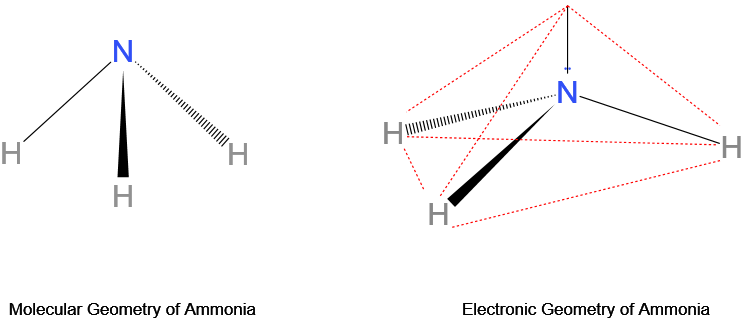
An example with differing molecular and electron geometries is that of ammonia, NHthree. With the primal cantlet nitrogen having 5 valence electrons, it possesses 3 bond pairs and a lone pair of electrons. Its molecular geometry is trigonal pyramidal while its electron geometry is tetrahedral.
Prerequisite concept(s):
- Lewis Structure (Representation of Valence Electrons in a molecule)
- Valence Shell Electron Pair Repulsion (VSEPR) Theory
Difference between molecular geometry and electron geometry
| Molecular geometry | Electron geometry |
| Molecular Geometry is the arrangement of atoms in a molecule, normally relative to a single central atom. | Electron Geometry is the arrangement of electron pairs around a central atom. |
| It excludes lone pairs in deciding the shape of a molecule, although repulsion from lone pair(south) is taken into account merely in bond angles. | It considers the presence of both bail pair(s) and lonely pair(s) of electrons in determining the shape. |
Conclusion of Electron geometry
Since electrons are always moving and their paths cannot be accurately figured, the system of electrons is described in terms of electron density distribution.
Electron geometry is determined past the number of electron pairs. The following table gives an idea of electronic geometry co-ordinate to the number of electron pairs.
Related Resource:
- Molecular geometry: A complete guide
- Naming molecular compounds
- Wedge and Dash in molecular geometry
Determination of Molecular geometry
The shape of a molecule is determined by the bonded atom, although this does not mean the shape itself is unaffected by the presence (repulsion) of lone pair(due south).
Molecular geometry includes geometrical parameters such as bail lengths, bail angles, and torsional angles that help determine the position of atoms besides as a molecule'south general shape.
It influences a substance's properties such every bit its reactivity, color, polarity, magnetism, biological action, and stage of affair.
Various techniques to make up one's mind molecular geometry include Raman spectroscopy, infrared spectroscopy, and microwave spectroscopy.
AXE Method
AXE method is an efficient tabular idea to represent molecular geometries. A represents the central cantlet, each X represents an atom bonded to A (or bond pair), and each Due east represents a alone pair on the central atom.
Below is the table of molecular geometries, arranged for dissimilar electron pairs:
Key Takeaway(s)
Concepts Berg
Are electron geometry and molecular geometry definitions the same?
The definitions of molecular geometry and electronic geometry are different. They differ as molecular geometry refers to the arrangement of atoms in a molecule around the key atom(due south), while electron geometry refers to the system of electron density around the fundamental atom(s).
What is the divergence between electronic geometry and molecular shape?
The difference between electronic geometry and molecular geometry/shape is the inclusion of lone pair(s) of electrons in determining the geometry of a molecule.
How to determine electron geometry?
Electron geometry can exist adamant by finding out the number of electron pairs, both bonding and not-bonding pairs around the central atom(s).
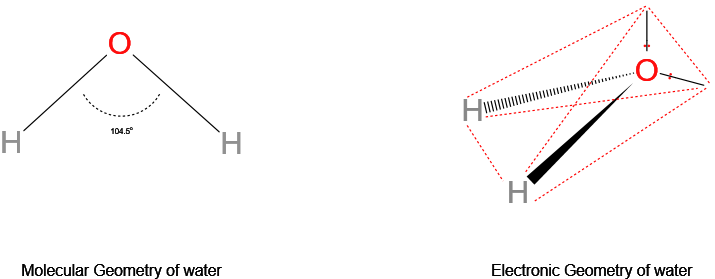
Show H2O Electron geometry vs molecular geometry.
The cardinal oxygen atom has 6 valence electrons, two of which form two bond pairs with the hydrogen atoms, and the other iv electrons grade ii alone pairs. This results in a tetrahedral electron geometry vs a bent molecular geometry.
What is the electron geometry of NHthree?
The electron geometry of ammonia (NH3) is tetrahedral because the central nitrogen cantlet, having 5 valence electrons, bonds three of its electrons with iii hydrogen atoms, and the remaining ii electrons act every bit a unmarried lone pair.
So there are a total of 4 electron pairs around the cardinal nitrogen cantlet. A tetrahedral electron geometry results, with an ∠HNH bond angle of 107° rather than 109.5° due to more repulsion from the alone pair as compared to a bond pair.
What is the electron geometry of COtwo?
The electron geometry of carbon dioxide, CO2, is linear because the fundamental carbon atom, having 4 valence electrons, forms a double bond with each of the oxygen atoms. The oxygen atoms arrange as far autonomously as possible with a bail angle of 180° between them. This results in a linear shape.
What is the electronic geometry of SO2?
The fundamental sulfur atom has 6 valence electrons and forms two double bonds with the two oxygen atoms, using 4 of its valence electrons. The remaining two valence electrons act equally a lone pair. With a total of 3 electron pairs effectually sulfur, the electronic geometry of sulfur dioxide, SO2 is trigonal planar. The ∠OSO bond angle is 119° instead of 120°, as the solitary pair causes more repulsion (than a bond pair).
What is the electron geometry for HiiS?
The electron geometry of HtwoDue south is tetrahedral as the central sulfur atom possesses 4 pairs of electrons; 2 of which are lonely pairs (4 electrons) and 2 are bond pairs with hydrogen atoms (two electrons), for sulfur's 6 valence electrons. The ∠HSH bond angle is 92° instead of 120°, with the lonely pairs causing more repulsion than the bond pairs.
What is the molecular geometry of H2S?
The molecular geometry of H2South is bent since the central sulfur atom has 2 lonely pairs (4 electrons) and ii bond pairs with hydrogen atoms (2 electrons) effectually it, for its 6 valence electrons. The ∠HSH bond angle is 92°.
Why is in that location a departure in the bail angles of HiiDue south and H2O?
The bail angle of HiiSouth is 92° while that of HiiO is 104.5°. This divergence is because oxygen is more electronegative and smaller in size than sulfur. Its electron density (of lone pairs) is less spread and so causes lesser repulsion on the bail pairs (more repulsion betwixt bond pairs) and then the bond pairs are farther apart from each other.
What is the molecular geometry for NO2, plus or minus?
The molecular geometry of NOii (+) is linear considering the key nitrogen cantlet has four valence electrons (positive accuse on N), forming a double bail with each of the oxygen atoms. In that location are only 2 bail pairs and no lone pairs, resulting in a 180° bond angle and linear geometry.
The molecular geometry of NO2 (-) is bent because, the primal nitrogen atom has 6 valence electrons (negative charge on N), four of which are used in forming two double bonds with oxygen atoms, and the remaining ii form a lone pair. This results in a bent geometry with a bond angle of 115°.
What is the molecular geometry of NCl3?
The molecular geometry of NCl3 is trigonal pyramidal because the central nitrogen atom has 5 valence electrons, iii of which course bond pairs with chlorine atoms, and the remaining two form a lone pair. A 103° bond angle is formed as a result.
What is the molecular geometry of SF4?
The molecular geometry of SF4 is seesaw-like, as the cardinal sulfur cantlet has 6 valence electrons, four of which course bond pairs with fluorine atoms, and the remaining 2 form a alone pair. The bond angles are 102° and 173°.
What is the difference between shape and geometry?
The shape of a molecule is the construction predicted using only bond pairs effectually the central atom (Molecular geometry) whereas the geometry of a molecule uses both bond pairs and solitary pairs in determining the structure (Electron geometry): but are often used interchangeably, especially when there are no lone pairs.
How is the molecular geometry of Oiii determined?
The molecular geometry of Ozone(O3) can be determined by knowing the electron pairs on the central oxygen cantlet. Having 5 valence electrons (positive formal charge on the fundamental oxygen), the central O atom forms a double bond with 1 of the terminal O atoms and a single bond with the other terminal O atom. It is likewise left with a lone pair of electrons (A miracle called resonance occurs).
As we know there are two bond pairs and a lone pair on the cardinal O atom, its molecular geometry is bent.
What is the molecular geometry of SiO2?
The molecular geometry of SiO2 is linear since the central silicon cantlet has 4 valence electrons, which are all used in forming ii double bonds with the oxygen atoms. The bond bending is 180°. Withal, this molecular form of silicon exists rarely and has been produced in an argon matrix.
The actual geometry of silicon dioxide, and SiOtwo is circuitous (polymer). It is a giant molecule in which silicon is surrounded past four oxygen atoms in a tetrahedral manner like a diamond. The bond angle is 97°. This arrangement is spread throughout the lattice.
Why is a six-bail molecular geometry called octahedral and not hexahedral?
It is because the number of triangular faces in a 6 bond molecular geometry is 8. The proper name of this geometry is decided past the number of faces of the octahedron shape formed by the zipper of half-dozen ligands. So, information technology cannot exist called hexahedron or six-hedron, but octahedron.
What is the electron group geometry of IFv?
The electron geometry of IFv is square pyramidal as the central iodine cantlet has v bond pairs (with the fluorine atoms) and a solitary pair for its 7 valence electrons. The bond angles are less than 90° because of more than repulsion from the lonely pair.
Why does NH3 have pyramidal shapes like H3O+ but NH4 + ion has a tetrahedral shape similar to CH4?
Because NH3, like H3O+, has 3 bond pairs and a lone pair on the primal atom. This results in trigonal pyramidal geometry. However, NHiv +, similar CH4, has four bail pairs and no alone pairs on the central atom, resulting in a tetrahedral shape
What is the molecular shape of IFiv (+) and IFiv (-)?
The molecular shape of IF4 (+) is seesaw-like, as the key iodine cantlet has 4 bond pairs (with fluorine atoms) and a lone pair of electrons for its half-dozen valence electrons (positive charge on iodine).
The molecular shape of IF4 (-) is square planar because the primal iodine cantlet in this case has four bond pairs and 2 lonely pairs for its viii valence electrons (negative accuse on iodine).
How do I evidence that SO3 ii- is trigonal pyramidal with VSEPR theory?
On the central sulfur atom, in sulfite ion, SO3 (2-), there are 6 valence electrons. 2 of these electrons are used in forming a double bond with one oxygen atom, and 2 more are used in forming two unmarried bonds with the other two oxygen atoms and the remaining two electrons course a single solitary pair. The negative charges prevarication on ii oxygen atoms and a miracle chosen resonance takes place.
three bail pairs and a lone pair result in trigonal pyramidal geometry, according to VSEPR theory.
Reference Book(s)
- Molecular geometry by Dr. Alison Rodger
Reference link(s)
- Molecular geometry (wikipedia.org)
Was this article helpful?
Electron Pair Geometry For Nh3,
Source: https://psiberg.com/difference-between-electron-geometry-and-molecular-geometry/
Posted by: elliottviaguld99.blogspot.com


0 Response to "Electron Pair Geometry For Nh3"
Post a Comment